

NuPulse Voyage™
The first minimally invasive, long-term cardiac
assist device that allows for ambulation and discharge home.
assist device that allows for ambulation and discharge home.
Working to transform the treatment of heart failure
The NuPulse Story

The Nupulse Voyage is the only minimally invasive heart pump that allows for both ambulation and discharge home. Both of these achievements were demonstrated in NuPulse’s 81 patient early feasibility study.
We Are Different in Our Approach
Although there are many minimally invasive heart pumps, some currently available, and many in various stages of development and clinical investigation, the Voyage is the only pump that works on the physiological principles of counterpulsation.
Every other pump under development is some type of rotary pump commonly known as a Pvad- a percutaneous ventricular assist device. Frequently, we are asked about the Abiomed Impella Pumps.
These are remarkable percutaneous pumps and serve a large unmet clinical need especially in the cardiogenic shock population.
Every other pump under development is some type of rotary pump commonly known as a Pvad- a percutaneous ventricular assist device. Frequently, we are asked about the Abiomed Impella Pumps.
These are remarkable percutaneous pumps and serve a large unmet clinical need especially in the cardiogenic shock population.
The Voyage is targeted at a different patient population,those patients with chronic heart failure.
The NuPulse device and the Abiomed devices are not competitive but complimentary
The NuPulse device and the Abiomed devices are not competitive but complimentary

The Idea For Long Term
Ambulatory Counterpulsation
Ambulatory Counterpulsation
NuPulse’s founders Doctors Val Jeevanandam and Douglas Althschuler have shared a keen interest in counterpulsation for many year.
They have both witnesses the clinical benefits counterpulsation provides for a large patient population.
Today, counterpulsation is delivered in hospitals using a device called the Intra-Aortic Balloon Pump- IABP.
Although the therapy of counterpulsation administered through the use of traditional IABP’s is beneficial to patients and is employed more than 50,000 times annually in the U.S. alone, the device has severe limitations.
The patient is tethered to a large 100 pound console and is confined to the ICU. They certainly cannot be discharged home.
They have both witnesses the clinical benefits counterpulsation provides for a large patient population.
Today, counterpulsation is delivered in hospitals using a device called the Intra-Aortic Balloon Pump- IABP.
Although the therapy of counterpulsation administered through the use of traditional IABP’s is beneficial to patients and is employed more than 50,000 times annually in the U.S. alone, the device has severe limitations.
The patient is tethered to a large 100 pound console and is confined to the ICU. They certainly cannot be discharged home.
The challenge has always been how do you deliver the life saving technology of counterpulsation but also allow patients to have the freedom to leave the ICU in a hospital and then be discharged home.
This problem has perplexed engineers for decades until now.
Others have tried to meet this challenge but have not been successful.
This problem has perplexed engineers for decades until now.
Others have tried to meet this challenge but have not been successful.
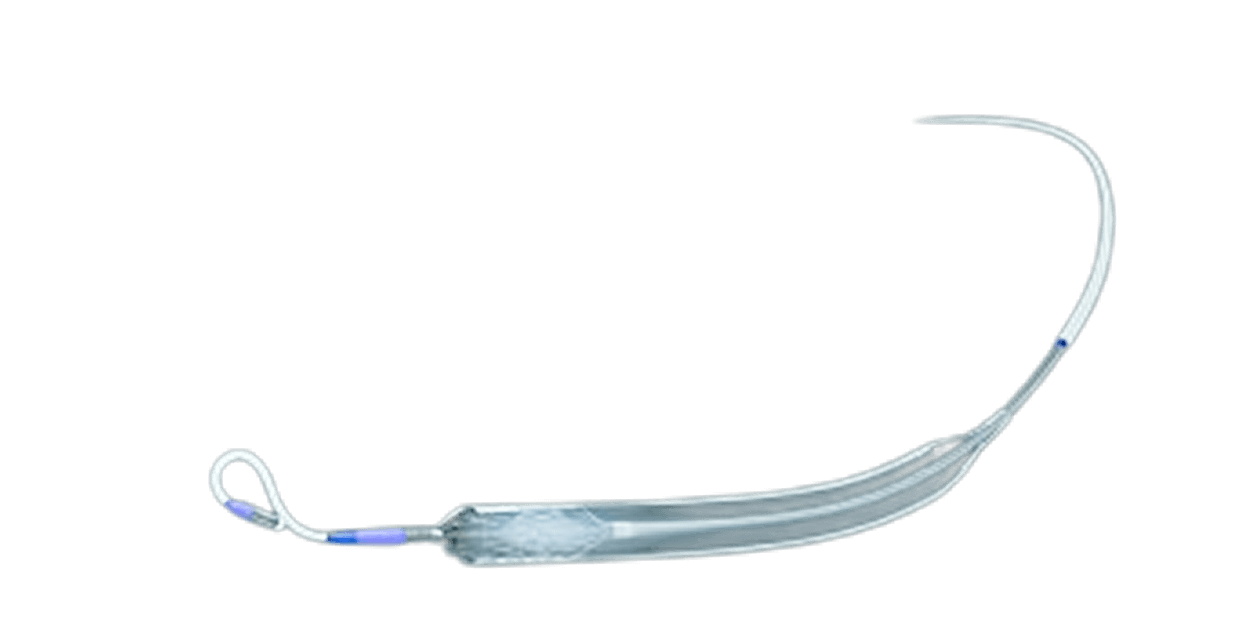



We Cracked the Code
NuPulse recruited a team of highly skilled and extremely dedicated engineers, scientists, and clinicians to develop the first successful ambulatory and long term counterpulsation device.
As shown in the illustration below the patient is no longer tethered to a 100-pound console but is able to carry a 5-pound portable driver around which allows complete and unrestricted movement.
As shown in the illustration below the patient is no longer tethered to a 100-pound console but is able to carry a 5-pound portable driver around which allows complete and unrestricted movement.
Patients are able to travel and take the Voyage of their dreams. Here is a photo of one of our sickest patients who was too ill prior to his Voyage Implant to travel.
After his Voyage implant he was able to finally visit, for the first time, his grandson in Europe.
After his Voyage implant he was able to finally visit, for the first time, his grandson in Europe.


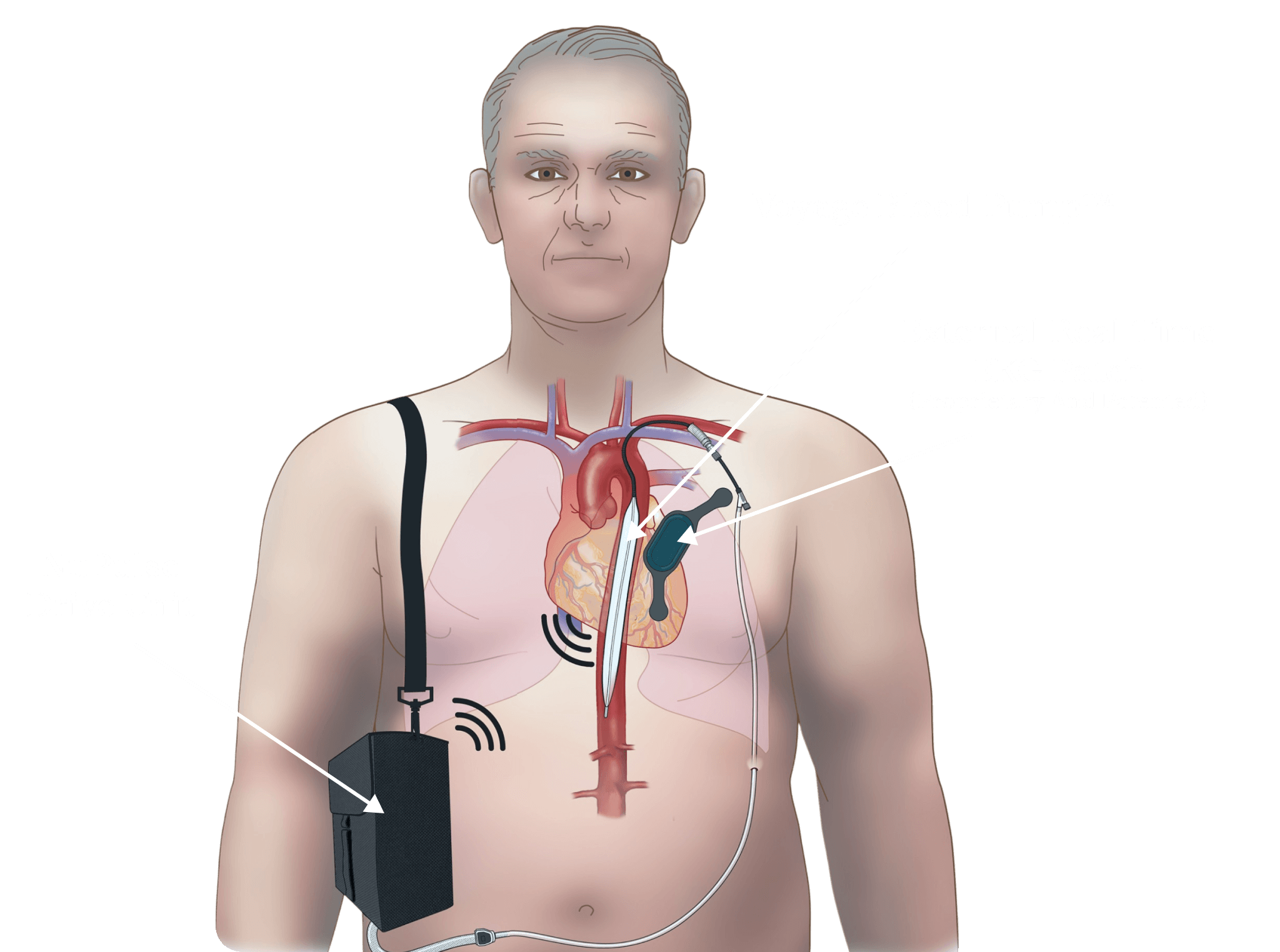
The Voyage operates on the same principles of counterpulsation as an intra-aortic balloon pump. A pump is placed in the descending aorta and inflated and deflated to help move blood through the body.
The pump is placed through an incision in the shoulder region of the patient and delivered through the axillary artery.
The pump is placed through an incision in the shoulder region of the patient and delivered through the axillary artery.
Caution-Investigational Device, Limited by United States Law to Investigational Use.
Not Available for Sale.

The Path Forward
NuPulse conducted an 81patient Early Feasbility Study where 3 distinct cohorts of patients were implanted. The first cohort were patients who were in the hospital who were listed as UNOS status 1 and urgently needed a heart. The second cohort weee those patients who needed a heart but had to clear up an underlying issue,for example the cessation of smoking or loosing weight. These patients were implanted. sent home and brought back once they were eligible for a transplant. The third cohort were all comers. These were gravely ill patients for whom there were no other options. They were told to go home ans get their affairs in order. To see a number if these patients thrive with their Voyage device and lice normal joyful lives was incredibly fufullong to the NuPulse Team.
The data from NuPulse’s 81-patient trial was submitted to the FDA which granted NuPulse approval to commence its final pivotal trial. This approval came in December 2019. Shortly thereafter the world shut down and approximately 6600 clinical trials were either suspended or not commenced. NuPulse took the latter path and instead of commencing enrollment in its pivotal trial used the Covid down time to further optimize the Voyage.
The data from NuPulse’s 81-patient trial was submitted to the FDA which granted NuPulse approval to commence its final pivotal trial. This approval came in December 2019. Shortly thereafter the world shut down and approximately 6600 clinical trials were either suspended or not commenced. NuPulse took the latter path and instead of commencing enrollment in its pivotal trial used the Covid down time to further optimize the Voyage.
Today the Voyage is a skin to skin procedure which we believe can be reliably performed in under 2 hours.
The company is now raising its final round of funding to finance the pivotal trial and, if necessary, have a limited commercial rollout.
We are partnering with the Cardiovascular Research Foundation - the premier CRO for implantable cardiac medical devices.
Together we look forward to a trial that demonstrates the unique clinical benefits of the Voyage.
Together we look forward to a trial that demonstrates the unique clinical benefits of the Voyage.



Final Pivotal Trial
We are now in the final stages of completing testing the second generation optimized Voyage and will be submitting this test data to the FDA for permission to complete oir final pivotal trial which,if successful, will lead to commercialization.
Virtually every week we hear from physicians around the world asking us when they will have a chance to either participate in a trial or be able to implant a patient in need of the Voyage
Virtually every week we hear from physicians around the world asking us when they will have a chance to either participate in a trial or be able to implant a patient in need of the Voyage
These inquiries are gratifying and demonstrate the clinical need of the Voyage, and would welcome hearing from you.

Final Round of Funding
The company is now raising its final round of funding to finance the pivotal trial and, if necessary, have a limited commercial rollout.
Our Intellectual Property
We have strong and vetted IP, both patent protected, with key patents going through 2040 and strong and well protected trade secrets
We have strong and vetted IP, both patent protected, with key patents going through 2040 and strong and well protected trade secrets
Patient Testimonials

The Voyage device has been successfully implanted in several patients including the following people. Hear their stories by clicking on their videos.
Rob's Testimonial
Rob Gentile participated in the Voyage Feasibility Study while he waited for his heart transplant. Watch the video to hear his story.
Pam’s Testimonial
Pam Morris-Walton participated in the Voyage Feasibility Study while she waited for her heart transplant. Watch the video to hear her story
Tom's Testimonial
Thomas Gnadt participated in the Voyage Feasibility Study while he waited for his heart transplant. Watch the video to hear his story
Karen’s Testimonial
Karen Seliga participated in the Voyage Feasibility Study while she waited for her heart transplant. Watch the video to hear her story
News & Events

